When your doctor prescribes a brand-name drug and your insurance pushes you toward a cheaper option, you might hear the terms authorized generic and branded generic thrown around like they’re the same thing. But they’re not. And the difference could matter more than you think - especially if you’re on a medication where even small changes in how it’s made can affect how it works in your body.
What Exactly Is an Authorized Generic?
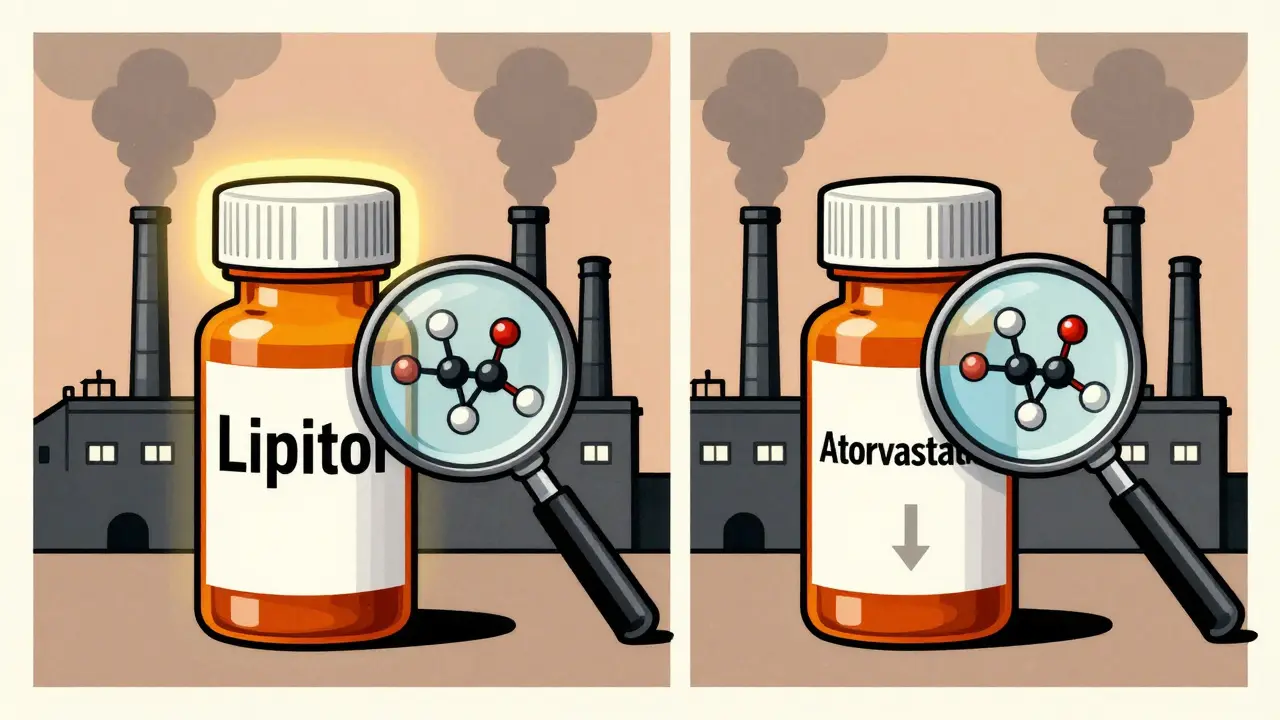
An authorized generic isn’t just another generic. It’s the exact same drug your doctor originally prescribed, just without the brand name on the bottle. The company that made the original brand-name drug - say, Pfizer with Lipitor - produces it themselves under a different label. Same pills. Same factory. Same inactive ingredients like fillers and coatings. The only thing that changes? The price tag.
This isn’t some loophole. The FDA defines it clearly: an authorized generic is an approved brand-name drug sold without the brand name. It uses the same New Drug Application (NDA) as the original. That means every single component - from the active ingredient to the tiny amount of lactose in the tablet - is identical. No guesswork. No reformulation. Just the same medicine you’ve been taking, now cheaper.
What About Regular Generics?
Regular generics, on the other hand, are made by different companies. They must contain the same active ingredient as the brand, but they’re allowed to use different inactive ingredients. That’s where things get tricky. For most drugs - like statins or blood pressure pills - those differences don’t matter. Your body absorbs the medicine the same way. But for some medications, even small changes in fillers or how the pill breaks down can cause problems.
Take lamotrigine, an epilepsy drug. A 2018 study in Neurology found that switching from brand to regular generic versions led to breakthrough seizures in some patients. Why? Because the inactive ingredients changed how the drug was absorbed. The same thing has been reported with thyroid meds like levothyroxine and immunosuppressants like cyclosporine. In these cases, the body is super sensitive to tiny variations. And that’s where authorized generics step in - they eliminate that risk entirely.
Cost Difference: It’s Not Just About Price
Here’s the catch: authorized generics cost more than regular generics. How much more? Usually 5-15% higher. Let’s break it down with real numbers.
For Concerta (methylphenidate ER), a common ADHD medication:
- Brand-name: $210 for 30 days
- Authorized generic: $185
- Regular generic: $165
That $20 difference might seem small, but if you’re paying out of pocket or have a high deductible, it adds up. Regular generics are often 80-85% cheaper than brand drugs. Authorized generics? They’re only 10-30% cheaper. So if cost is your main driver, regular generics win. But if you’ve had bad reactions to generics before - nausea, dizziness, loss of effectiveness - then that extra cost might be worth it.

Real Patient Experiences
People aren’t just guessing. They’re sharing what works - and what doesn’t.
One Reddit user with epilepsy wrote: "Switched from brand Lamictal to a regular generic. Two seizures in three weeks. Switched back to the authorized generic - zero issues for 18 months."
Another person managing high blood pressure said: "Went from brand Lisinopril to a regular generic. My BP actually improved. Saved $400 a month. No side effects."
A 2023 GoodRx survey of 5,000 users found that 18.7% had problems switching to regular generics. The top complaints? Gastrointestinal issues (42%), feeling like the drug didn’t work as well (33%), and new side effects (25%). But only 6.2% of people who switched to authorized generics reported similar issues.
On Drugs.com, authorized generics average a 7.8/10 satisfaction rating. Regular generics? 7.2/10. Not a huge gap - but for people with chronic conditions, that 0.6-point difference can mean the difference between feeling stable and feeling off.
When Should You Choose Authorized Over Regular?
Not every drug needs this level of precision. But for some, it’s critical. Here’s when to lean toward authorized generics:
- Narrow therapeutic index drugs: Where the difference between a dose that works and one that’s toxic is tiny. Think warfarin, lithium, digoxin, levothyroxine, and antiepileptics.
- Complex delivery systems: Extended-release pills, patches, inhalers, or injectables. These rely on precise manufacturing. A change in coating or particle size can alter absorption.
- History of switching problems: If you’ve had side effects or loss of effectiveness after switching to a regular generic before, stick with the authorized version.
- Chronic conditions with high stakes: If your health depends on rock-solid consistency - like heart failure, epilepsy, or transplant rejection - the extra cost may be a small price to avoid hospital visits.
For common drugs like ibuprofen, metformin, or sertraline? Regular generics are almost always fine. The science backs it up. The FDA’s bioequivalence standards (80-125% absorption range) are strict. For most people, the difference is negligible.
How to Spot the Difference at the Pharmacy
Here’s the problem: most pharmacies don’t make it easy. The label won’t say "authorized generic." You might just see "methylphenidate ER" with a different company name.
Ask your pharmacist for the National Drug Code (NDC). Authorized generics usually share the same manufacturer code as the brand. For example, if the brand is made by Janssen, the authorized generic will also say Janssen - just without the brand name. Regular generics will show a different manufacturer entirely.
Some pharmacies now label authorized generics with a small note: "Manufactured by the brand company." But not all do. Don’t assume. Ask.
What Your Insurance Might Say
Insurance companies love regular generics. They’re cheaper. So they often put them on the lowest tier - meaning you pay the least. Authorized generics? They’re sometimes classified as "brand equivalent," which means you pay more. You might even get a higher copay.
Check your plan’s formulary. If the authorized generic isn’t covered or requires prior authorization, you’ll need to talk to your doctor about writing a "do not substitute" note. Some insurers are starting to change this. Thanks to the 2022 Consolidated Appropriations Act, Medicare Part D plans now have to tell you if an authorized generic is available. That’s a step forward.
The Bottom Line: It’s Not One-Size-Fits-All
There’s no universal answer. For most people, regular generics are safe, effective, and save money. But for others - especially those on critical medications - the authorized generic is the smarter, safer choice.
If you’ve ever felt "off" after switching to a generic - even if your doctor said it was fine - trust that feeling. Talk to your pharmacist. Ask for the NDC. Check if an authorized version exists. And if cost is a barrier, ask your doctor about samples or patient assistance programs. You’re not overreacting. You’re being smart.
The goal isn’t to avoid generics. It’s to avoid unnecessary risk. And sometimes, paying a little more for the exact same medicine is the best way to stay healthy.
Are authorized generics as safe as brand-name drugs?
Yes. Authorized generics are made by the same company, in the same facility, with the exact same ingredients as the brand-name drug. The only difference is the label. The FDA considers them therapeutically identical. If you’ve had no issues with the brand, you won’t have any with the authorized generic.
Can I ask my pharmacist to give me an authorized generic instead of a regular one?
Yes - but you have to ask. Pharmacists are allowed to substitute regular generics unless your doctor writes "dispense as written" or "do not substitute." To get an authorized generic, you need to specifically request it. Don’t assume they’ll offer it. Bring up the name of the brand drug and ask if the authorized version is available.
Why aren’t authorized generics more common if they’re so similar to brand drugs?
Because they cost more than regular generics. Brand companies make authorized generics to compete with cheaper generics after patent expiration, but they don’t want to undercut themselves too much. So they price them just below the brand, not below the regular generic. Most insurers and pharmacies prefer the cheaper option, so authorized generics are often overlooked unless you specifically ask for them.
Do authorized generics show up in the FDA’s Orange Book?
No. The Orange Book lists therapeutically equivalent generic drugs that went through the Abbreviated New Drug Application (ANDA) process. Authorized generics are marketed under the original brand’s NDA, so they’re not listed there. That’s why it’s harder to find them - you have to check the manufacturer or ask your pharmacist.
Is it worth switching from a regular generic to an authorized generic if I’m doing fine?
If you’re stable, with no side effects or loss of effectiveness, switching isn’t necessary. But if you’re paying out of pocket and the authorized version is only slightly more expensive, it might be worth it for peace of mind. For high-stakes medications like epilepsy or thyroid drugs, even small changes can matter. If you’ve never had issues, stick with what works. But if you’ve ever had a bad reaction, it’s worth exploring the authorized option.



Esha Pathak
February 15, 2026 AT 19:27Okay but like… imagine your brain is a delicate origami crane and every generic is a different fold 🤷♀️ Authorized generics? Same paper, same artist, same patience. Regular generics? Some guy in a warehouse with a stapler trying to replicate it. I switched to authorized lamotrigine after my third seizure in six months. Now I’m back to painting, hiking, and not crying in the shower. Money is a factor, sure - but so is your damn life.
Mike Hammer
February 17, 2026 AT 01:04Yea i’ve been on generic adderall for 3 years. no probs. then switched to authorized and honestly? felt like i got my focus back. not a huge difference but like… the edge was sharper? idk. maybe placebo. but if it works, why not? also pharmacy kept giving me the wrong generic and i didn’t even know till i checked the ndc. mind blown.
Charlotte Dacre
February 17, 2026 AT 17:11Oh honey. You’re telling me insurance companies are *still* pushing the cheapest thing on the shelf like it’s a clearance sale at Walmart? 🙄 Let me guess - they don’t care if you end up in the ER because your thyroid decided to go on vacation. Meanwhile, the authorized generic costs $185 instead of $165. That’s 12 bucks. For a month. That’s less than your weekly coffee habit. Priorities, people.
Virginia Kimball
February 19, 2026 AT 06:53THIS. I’ve been on levothyroxine for 12 years. Switched to a regular generic once because my insurance forced it. Suddenly I was exhausted, gaining weight, and my heart was racing at 3 a.m. Went back to the authorized version - same manufacturer, same pill, just different label - and boom. Back to normal. I don’t care if it costs $10 more. My body isn’t a lab experiment. Also, pharmacists? Ask them for the NDC. They’ll know. Don’t be shy.
Betty Kirby
February 21, 2026 AT 00:55People who panic about generics are just lazy. The FDA tests these things. If it’s bioequivalent, it’s bioequivalent. Stop paying extra for a label. You’re not special. Your thyroid doesn’t need a VIP pass. This whole "authorized generic" trend is just Big Pharma’s way of milking you again. Get over it.
Sarah Barrett
February 22, 2026 AT 18:02It’s worth noting that the FDA’s bioequivalence standards allow for a 80–125% absorption range. That’s a 45% window. For most drugs, fine. For narrow-therapeutic-index drugs? That’s like flying a 747 with a 30% variance in fuel-to-air ratio. The authorized generic eliminates that variance entirely. It’s not about fear - it’s about precision. And in medicine, precision saves lives.
Erica Banatao Darilag
February 23, 2026 AT 21:51i just wanted to say thank you for this post. i’ve been on cyclosporine since my transplant and switched to a regular generic once because my insurance said "it’s the same." i got sick. real sick. took 3 weeks to recover. now i only take the authorized version. i didn’t know it was even an option. i’m so glad someone explained how to ask for it. i’ll be telling my whole support group.
Daniel Dover
February 24, 2026 AT 19:48Authorized generic = same pill. Regular generic = different filler. For some drugs, that filler changes how fast it hits your bloodstream. Simple. Stop overcomplicating it.
Chiruvella Pardha Krishna
February 25, 2026 AT 08:00When I was in medical school in Delhi, we called these "ghost generics" - the ones that look like the real thing but carry the soul of the original. The authorized version is not a copy. It is the original, wearing a mask. And in a world where everything is counterfeit - from watches to medicines - to choose the mask that carries the same fingerprint? That is wisdom.
Kapil Verma
February 25, 2026 AT 21:32Why are Americans so obsessed with paying more? In India, we use generics because they’re proven. If your body can’t handle a generic, maybe your body is weak. This "authorized" nonsense is just capitalism exploiting fear. Get over it. We don’t need your expensive labels here.