When you hear someone say they got tested for cancer risk, most people think of mammograms or colonoscopies. But there’s another layer-something hidden in your DNA-that can tell you far more about your future health than any scan ever could. Genetic testing for inherited cancer risk isn’t science fiction anymore. It’s here, it’s accurate, and for some people, it’s life-saving.
What BRCA and Lynch Really Mean
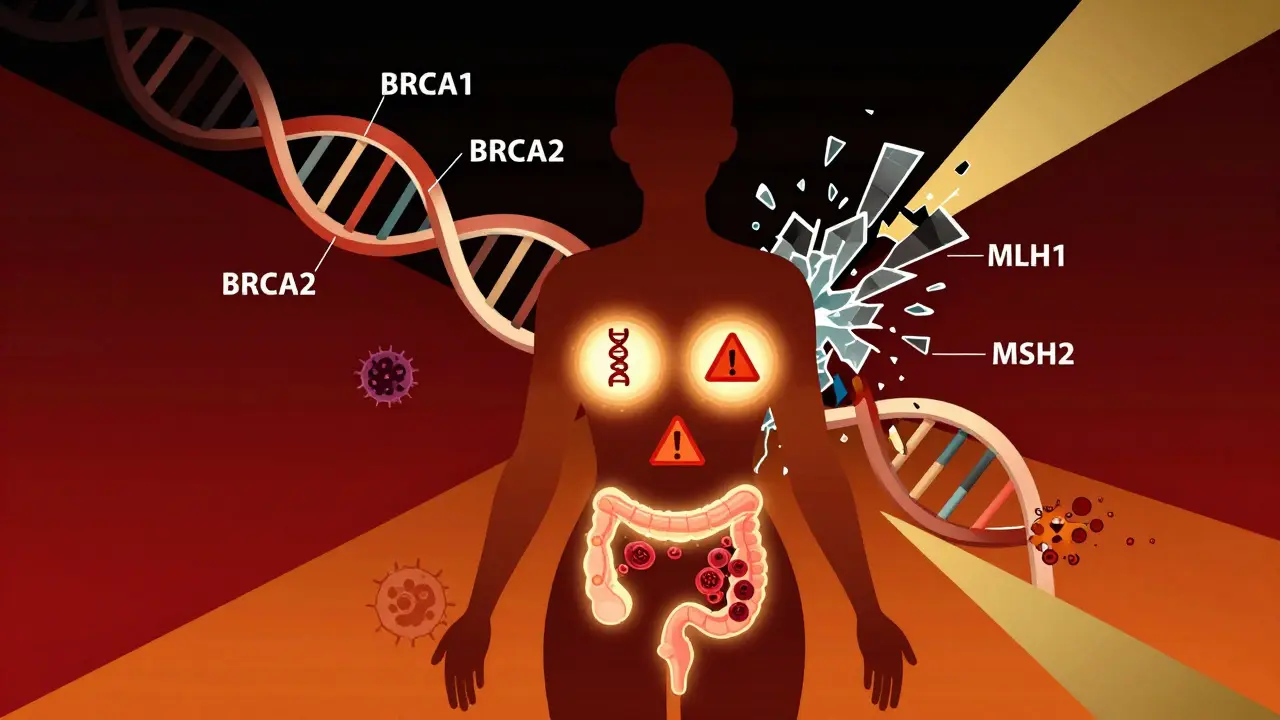
BRCA1 and BRCA2 aren’t just random letters. They’re genes that normally help fix broken DNA. When these genes are mutated, cells can’t repair damage properly. That’s when cancer starts to creep in. Women with a harmful BRCA1 mutation have up to a 72% chance of developing breast cancer by age 80. For BRCA2, it’s around 69%. Ovarian cancer risk jumps to 44% for BRCA1 and 17% for BRCA2-compared to less than 2% in the general population.
Lynch syndrome is different. It’s not about breast or ovarian cancer primarily. It’s about your colon. Mutations in MLH1, MSH2, MSH6, PMS2, or EPCAM genes mean your body loses its ability to fix errors during cell division. That leads to colorectal cancer-sometimes before age 40. Lifetime risk? Up to 80% depending on which gene is broken. Endometrial cancer risk also skyrockets, especially in women.
These aren’t rare. About 1 in 400 people carry a BRCA mutation. Lynch syndrome affects roughly 1 in 280. Most people don’t know they have it until someone in the family gets cancer young. That’s why testing isn’t just for those already diagnosed-it’s for people with a family history, even if they feel fine.
How Testing Works Today
Forget testing just one gene at a time. In 2025, the standard is multigene panel testing. A single blood or saliva sample can check 60 to 80 genes linked to cancer risk. This includes BRCA1/2, Lynch genes, plus others like PALB2, ATM, CHEK2, and RAD51C-genes that were only discovered in the last decade.
The tech behind it is powerful. Next-generation sequencing reads your DNA letter by letter, catching tiny changes most older tests would miss. Labs now hit 99.5% accuracy on single-letter mutations. Results come back in two to three weeks. But here’s the catch: not every change is clear-cut.
That’s where VUS-variants of uncertain significance-come in. A few years ago, nearly 1 in 8 people got a VUS result. It meant: “We found something odd, but we don’t know if it’s dangerous.” That caused panic, confusion, and unnecessary surgeries. But in February 2025, Mayo Clinic published a breakthrough. Using CRISPR to test nearly 7,000 BRCA2 variants in the lab, they reclassified 91% of previously unclear changes. For the DNA-binding region of BRCA2, VUS rates dropped from 12.7% to just 1.1%. That’s huge. It means fewer people are left guessing.
Why Panel Testing Beats Single-Gene Tests
Some clinics still offer BRCA-only testing. It’s cheaper, faster, and has fewer VUS results. But here’s the problem: you’re missing half the picture.
A 2023 study of over 38,000 people found that single-gene BRCA testing missed 30% to 50% of actionable mutations. Someone might test negative for BRCA but carry a harmful PALB2 mutation-which raises breast cancer risk nearly as high as BRCA. Or they might have Lynch syndrome and never know it until they’re diagnosed with stage 3 colon cancer.
Comprehensive panels catch those. They also find mutations in genes linked to pancreatic, prostate, and melanoma cancers. That’s critical. If you know you carry a mutation in a gene tied to multiple cancers, your screening can be tailored. For example, someone with a CHEK2 mutation might start colonoscopies at 40 instead of 50. A person with ATM might get annual breast MRIs earlier.
Whole-genome sequencing sounds impressive-it checks your entire DNA. But it finds so many unclear changes (14.7% VUS rate) that it often creates more confusion than clarity. And it costs over $1,800. For most people, a targeted panel of 60+ genes is the sweet spot: high accuracy, lower cost ($250-$500 out-of-pocket), and actionable results.
What Direct-to-Consumer Tests Get Wrong
You’ve seen the ads: “Test for cancer risk from your kitchen.” Companies like 23andMe offer FDA-authorized BRCA testing. But here’s the truth: their test only looks for three specific mutations common in people of Ashkenazi Jewish descent. That’s it.
For everyone else-non-Jewish, non-Ashkenazi populations-it misses over 97% of harmful BRCA mutations. A 2024 study in the New England Journal of Medicine showed that out of 1,000 people with true BRCA mutations, 970 would get a false negative from 23andMe. That’s not prevention. That’s false reassurance.
And it doesn’t test for Lynch syndrome at all. No PALB2. No ATM. Nothing beyond those three variants. If you’re relying on a DTC test for cancer risk, you’re playing Russian roulette with your health.
Who Should Get Tested?
The National Comprehensive Cancer Network (NCCN) has clear guidelines. You should consider testing if:
- You were diagnosed with breast cancer before age 45
- You have ovarian, pancreatic, metastatic prostate, or male breast cancer in your family
- Two or more close relatives have breast, ovarian, or colorectal cancer
- You’re of Ashkenazi Jewish descent with any family history of these cancers
- You’ve had colorectal or endometrial cancer before age 50
Even if you don’t have cancer yourself, a family history matters. A cousin with ovarian cancer? A grandfather who died of colon cancer at 42? Those count.
And here’s something new: in 2025, major cancer centers are starting to test all patients diagnosed with certain cancers-like ovarian, pancreatic, or metastatic prostate-for inherited mutations. Why? Because if you have a BRCA mutation, you might respond better to certain drugs like PARP inhibitors. Your treatment changes. Your family’s future changes too.
The Real Cost and Insurance Coverage
Cost used to be a barrier. Now? Medicare covers testing if you meet NCCN criteria-and approves 98.7% of requests. Most private insurers follow suit. Out-of-pocket costs? Often $0 if you qualify. If you don’t qualify, self-pay panels run $250-$500. That’s less than a single MRI.
But there’s a hidden cost: reclassification. If your test shows a VUS, and later it’s reclassified as harmful, you might need a follow-up test. Myriad Genetics charges $250 for this update. Other labs offer it free. Always ask upfront.
And yes, insurance discrimination still happens. GINA protects you from health insurance discrimination based on genetic results-but not life, disability, or long-term care insurance. A 2024 study found 9% of people reported being denied coverage after genetic testing. Know your rights.
What Happens After a Positive Result?
A positive result isn’t a death sentence. It’s a heads-up.
For BRCA carriers:
- Start breast MRI and mammograms at age 25-30
- Consider risk-reducing mastectomy-studies show it cuts breast cancer risk by up to 95%
- Remove ovaries and fallopian tubes by 35-40 (reduces ovarian cancer risk by 80% and breast cancer risk by 50%)
For Lynch syndrome:
- Colonoscopy every 1-2 years starting at 20-25
- Consider aspirin daily (shown to reduce colorectal cancer risk by 60% in Lynch patients)
- Endometrial biopsies for women, starting at 30-35
And here’s the win: if you have Lynch syndrome and get colorectal cancer, immunotherapy like pembrolizumab works far better than traditional chemo. One 2025 case study at Fred Hutchinson showed complete remission in a 42-year-old with stage 3 colon cancer-because they knew to test for Lynch first.
The Human Side: Anxiety, Family, and Truth
Testing changes more than your medical plan. It changes your family.
One woman in Perth tested positive for BRCA2 at 28. She had no cancer. But she had a 69% risk. She chose prophylactic surgery. On a Reddit thread, 82% of people with her experience said it eased their anxiety more than any medication could.
But not everyone feels that way. Some get a VUS and spiral into fear. Others test negative and assume they’re safe-only to develop cancer later from a gene not on the panel. A 2024 JAMA study documented three patients who declined surgery after a “negative” test, then developed cancer from an undetected mutation.
That’s why genetic counseling isn’t optional. It’s essential. A certified counselor helps you understand what the results mean, what your options are, and how to talk to your family. Most insurance covers it. Most cancer centers now require it.
And yes, the system isn’t perfect. Community clinics still lag behind academic centers. Black patients are tested at less than half the rate of white patients. But progress is real. In 2025, 87% of U.S. cancer centers have integrated genetic testing into their electronic records. Results flow automatically to doctors. No more lost paperwork.
The Future: Beyond Single Genes
What’s next? Polygenic risk scores. Researchers at Stanford identified 380 DNA variants that control how genes turn on and off across 13 cancers. These aren’t single mutations-they’re tiny tweaks, hundreds of them, working together. When combined, they can predict cancer risk with surprising accuracy.
In the next decade, your genetic risk report might say: “Your BRCA1 mutation raises your breast cancer risk by 70%. Your 380 other variants add another 20%. Total risk: 90%.” That’s not science fiction. It’s coming.
But for now, the best tool is still the panel test: BRCA, Lynch, PALB2, ATM, CHEK2-all in one. It’s accurate. It’s affordable. And if you’re at risk, it could save your life-or the lives of your children.
What to Do Next
If you have a family history of cancer, don’t wait. Talk to your doctor. Ask for a referral to a genetic counselor. Bring your family history-names, ages, types of cancer. Don’t rely on memory. Write it down.
If you’ve already had cancer, ask: “Could this be inherited?” Even if you’re past treatment, knowing your genetic risk helps your family. It might even change your next treatment.
And if you’ve done a DTC test? Don’t trust it. Go to a clinic. Get a proper panel. Your DNA doesn’t lie. But cheap tests sure can mislead you.





Danielle Stewart
December 18, 2025 AT 17:40My sister tested positive for BRCA2 last year. She was 31, no cancer, just a strong family history. We cried for hours. Then she had the surgery. Now she sleeps through the night. I know it sounds extreme, but if you’re sitting on a ticking clock and you can silence it? Do it. No regrets.
Genetic counseling saved her life-not just the test. Don’t skip that step.
Glen Arreglo
December 19, 2025 AT 13:32People act like this is all new, but my dad’s side has had Lynch syndrome since the 80s. We started colonoscopies at 20. My cousin got polyps at 22, removed them, still alive at 45. Meanwhile, my uncle waited till he was 48 and got stage 4. The difference? Awareness.
Testing isn’t fear-mongering. It’s legacy-building. You’re not just protecting yourself-you’re protecting your nieces, your nephews, your future kids. This isn’t optional if you’ve got a family tree with cancer branches.
shivam seo
December 19, 2025 AT 15:37Wow. Another overhyped medical article. BRCA? Lynch? Newsflash: most cancers are caused by smoking, obesity, and bad lifestyle. DNA doesn’t care how much you stress or how many processed meals you eat. You’re blaming genes to avoid responsibility.
And $500 for a test? That’s just another way for labs to profit off fear. Get a job. Eat veggies. Stop buying into biotech marketing.
benchidelle rivera
December 19, 2025 AT 15:43Shivam SEO is right about one thing: lifestyle matters. But you’re wrong to dismiss genetics. I’m a medical oncologist. I’ve seen patients with perfect diets and zero smoking history get metastatic breast cancer at 34 because of a PALB2 mutation.
Genetic testing doesn’t replace healthy living-it complements it. A woman with ATM doesn’t need to stop eating sugar. She needs a breast MRI at 30. That’s not fear. That’s precision medicine.
And yes, the VUS problem was real. But the Mayo Clinic breakthrough? That’s the kind of science that deserves applause, not cynicism.
Matt Davies
December 20, 2025 AT 07:23Let me tell you about my auntie. She got the 23andMe test, saw ‘no BRCA mutations,’ and thought she was golden. Two years later, she was diagnosed with stage 3 endometrial cancer. Turns out she had Lynch syndrome. 23andMe didn’t test for it. Not even close.
It’s like buying a car alarm that only works if someone tries to open the trunk. Meanwhile, the thief walks in through the front door.
Don’t be that person. If your family’s got cancer history, go to a clinic. Not an app.
Alana Koerts
December 20, 2025 AT 09:09DTC tests are useless. End of story.
pascal pantel
December 20, 2025 AT 17:39Let’s cut through the noise. The NCCN guidelines are outdated. They still rely on family history thresholds that were established in the 1990s. We now have polygenic risk scores that can stratify risk in asymptomatic individuals without any family history.
And the $250 panel? That’s a bargain. My lab charges $1,200 for a 120-gene panel with CNV and methylation analysis. The $250 ones? They miss structural variants. You’re getting a 70% solution and calling it a win.
Also, GINA? That’s a joke. Life insurance companies use ancestry and surname data to infer genetic risk. You think they don’t have algorithms for that? They do. And they’re not regulated.
Gloria Parraz
December 21, 2025 AT 06:51I got my results last month. VUS on CHEK2. I spent six weeks Googling. I had panic attacks. I cried in the shower. Then I found a genetic counselor who explained it like this: ‘We don’t know if it’s dangerous. But we know how to watch you. And we’ll update you if anything changes.’
That was the moment I stopped being scared and started being proactive.
If you’re reading this and you’re in the VUS spiral? Call your clinic. Ask for counseling. You’re not alone. And it’s not your fault. The science just hasn’t caught up yet.
Sahil jassy
December 23, 2025 AT 04:48bro just get tested if u have family history no cap
my uncle had colon cancer at 38
we all got tested
my sister has lynch
now she gets colonoscopy every year
she alive at 32
thank god we did it
Kathryn Featherstone
December 24, 2025 AT 09:46I’m a nurse in oncology. I’ve seen too many patients come in after a diagnosis, only to learn their cancer was inherited-and their siblings never got tested.
One woman came in with metastatic ovarian cancer at 36. Her sister had tested negative for BRCA years ago because they only tested BRCA1. Turns out she had BRCA2. Her sister didn’t know until it was too late.
Panel testing isn’t a luxury. It’s a responsibility. If you’ve got a family history, get the full panel. Don’t let outdated testing be the reason someone dies.
Nicole Rutherford
December 24, 2025 AT 11:38Everyone’s acting like this is some miracle breakthrough. Newsflash: 90% of people who get tested don’t even have a mutation. And the ones who do? They get scared, have unnecessary surgeries, and then their kids grow up terrified of their own DNA.
You think your 12-year-old daughter needs to know she has a 70% chance of breast cancer? That’s not prevention. That’s trauma.
And don’t even get me started on the ‘aspirin for Lynch’ thing. That’s a band-aid on a bullet wound. Real medicine isn’t about taking pills to delay cancer. It’s about not getting it in the first place.
Stop selling fear as science.