More than 90% of prescriptions filled in the U.S. today are for generic drugs. Yet, many doctors still hesitate to prescribe them-sometimes because patients ask, sometimes because they’re unsure themselves. If you’re a prescriber wondering whether generics are truly the same, or how to explain that to a skeptical patient, you’re not alone. The good news? Clear, science-backed resources exist to help you make confident, cost-effective decisions without sacrificing care.
What Makes a Generic Drug the Same as a Brand-Name Drug?
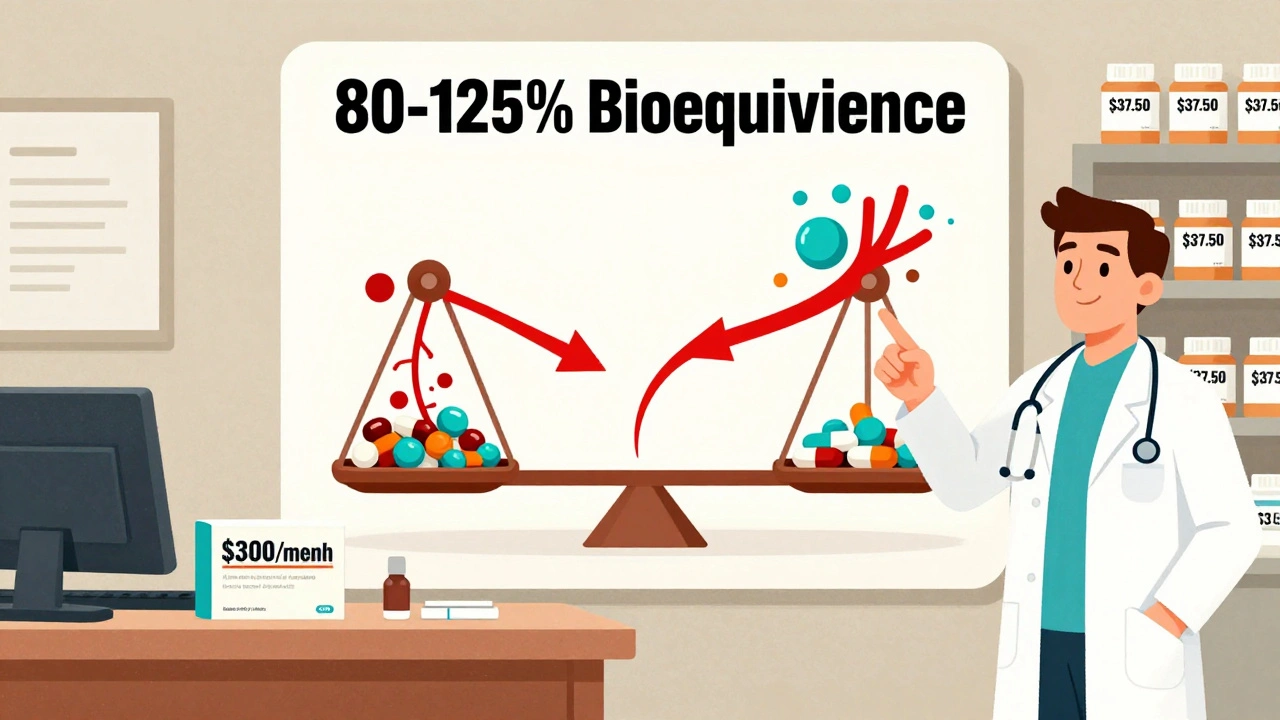
A generic drug isn’t a copy. It’s not a cheaper version with less active ingredient. It’s the exact same medicine, approved by the FDA under strict standards. To get approval, a generic must deliver the same amount of active ingredient into the bloodstream at the same rate as the brand-name version. That’s called bioequivalence-and it’s measured within an 80% to 125% confidence interval. In plain terms: if the brand drug gets 100 units of medicine into your blood over time, the generic must get between 80 and 125 units. That’s not a wide range-it’s tight enough to ensure identical therapeutic effect.
The FDA requires this testing in 24 to 36 healthy volunteers. The same manufacturing rules apply: quality control, purity, stability, and labeling. The only differences? The inactive ingredients (like dyes or fillers) and the packaging. Those don’t affect how the drug works. And yes-generics are tested for the same side effects, same risks, same benefits.
Here’s what the data says: in 2022, the FDA analyzed over 12,000 adverse event reports for generic drugs. The number for brand-name drugs? Just under 12,000. No difference in safety. No difference in effectiveness. Just a big difference in cost.
Why Doctors Still Hesitate-And What’s Behind the Misconceptions
Despite the science, myths persist. Some doctors worry about variability between generic manufacturers. Others remember a time when generics were less reliable-before the Generic Drug User Fee Amendments (GDUFA) of 2012. That law gave the FDA more funding and authority to inspect overseas facilities and enforce standards. Today, the same plants that make brand-name drugs often make generics-just under different labels.
Patient concerns play a big role too. A 2021 FDA survey found 42% of Hispanic patients believed generics were less effective. Patients with incomes under $25,000 are 3.7 times more likely to skip meds because of cost. If you don’t address those fears directly, they’ll stick. And if you don’t have a simple way to explain bioequivalence during a 10-minute visit, you’re more likely to default to the brand.
One study showed physicians who received structured education on generics were 2.3 times more likely to start conversations about cost with patients. That’s not just about prescribing-it’s about trust. When patients feel you’re helping them save money without sacrificing care, they’re more likely to stick with the treatment.
Key Resources Doctors Can Actually Use
The FDA’s Generic Drugs Stakeholder Toolkit is the most comprehensive set of prescriber resources available. It includes:
- 12 ready-to-use social media templates to share with patients
- 5 customizable information cards you can print and hand out
- 3 infographics, including one titled What Makes a Generic the Same as a Brand-Name Drug?-a visual breakdown of the 80-125% bioequivalence range
The Prescriber Flyers (available in English and Spanish) are designed to fit in office waiting room racks. Version 2, updated in March 2022, includes QR codes that link directly to Spanish-language patient materials. These aren’t academic papers-they’re practical tools built for real clinics.
For complex drugs like inhalers, topical creams, or injectables, the FDA has separate guidance. These are harder to replicate exactly, and therapeutic equivalence isn’t always guaranteed. But for most oral medications-antibiotics, statins, blood pressure pills, antidepressants-the science is clear. Generics work the same.
How to Talk to Patients About Switching to Generics
Patients don’t need a lecture on pharmacokinetics. They need reassurance. Here’s what works:
- “This generic has the same active ingredient and works the same way.” Avoid saying “it’s the same thing.” Say “it works the same way.”
- “Your insurance is requiring this switch because it’s much cheaper.” Don’t frame it as a cost-cutting move by the system-frame it as a benefit to them.
- “The FDA requires this generic to be just as safe and effective as the brand.” Cite the agency. It’s trusted.
- “I’ve prescribed this generic to hundreds of patients. No one has had a problem.” Personal experience builds trust.
The FDA even provides sample scripts for tough conversations. For example: “My insurance wants me to switch, but my doctor said the brand works better.” The response? “I understand your concern. The FDA requires generics to meet the same standards. The difference isn’t in how well it works-it’s in the price.”
Where the System Still Falls Short
These tools are powerful-but they’re not built into most doctors’ daily workflows. Only 37% of major EHR systems (like Epic or Cerner) show pop-up reminders about generic alternatives during prescribing. That’s a missed opportunity. Imagine if, when you typed in “Lipitor,” your system automatically showed: “Generic atorvastatin available. Saves patient $262.50/month. Therapeutic equivalence: 99.7%.”
That’s not science fiction. Kaiser Permanente did it in 2021. They integrated FDA-approved generic information directly into Epic. Within six months, brand-name prescribing dropped by 18.7%. That’s real impact.
Another gap? Time. A 2022 study found 73% of physicians said they simply don’t have time to look up generic info during a visit. The solution? Embed it. Print the flyers. Save the infographics as bookmarks. Train your staff to hand them out. Make it part of the intake process.
What the Data Says About Cost and Adherence
Cost isn’t just a number-it’s a barrier to health. The American College of Physicians found that 20-30% of new prescriptions are never filled because of price. That’s not patient noncompliance-it’s economic reality.
Switching from a $300/month brand-name drug to its generic saves patients an average of $262.50 per month. That’s over $3,150 a year. For someone on a fixed income, that’s rent. That’s groceries. That’s keeping the lights on.
And adherence? Patients who get generics are more likely to stick with their treatment. A 2023 study showed that for every 10% increase in generic prescribing, medication adherence rose by 6%. That’s not just savings-it’s better outcomes. Fewer hospitalizations. Fewer complications. Fewer emergency visits.
What’s Changing in 2025 and Beyond
The FDA launched an API pilot in July 2023 that connects generic drug data directly to EHR systems. Early results show a 15.2% increase in generic prescribing among participating doctors. That’s just the start.
Medicare’s 2024 proposed rule includes financial incentives for plans that provide prescriber education on therapeutic alternatives. That means more funding, more training, more tools coming your way.
And AI is stepping in. IBM Watson Health tested a system that generates personalized generic substitution recommendations based on patient history and concerns. In a trial with 120 physicians, acceptance rates jumped by 29 percentage points. The future isn’t just about education-it’s about automation that supports, not replaces, your judgment.
Meanwhile, 44 states now have laws requiring pharmacists to substitute generics unless the prescriber says no. That means more patients will walk in asking for the generic. You need to be ready to answer.

How to Get Started Today
You don’t need to overhaul your practice. Start small:
- Download the FDA’s Prescriber Flyer and Generic Drug Facts Handout. Print one copy. Keep it on your desk.
- Next time a patient asks about a brand-name drug, say: “There’s a generic version. It works the same and saves you over $250 a month. Want me to switch it?”
- Ask your EHR vendor if they offer FDA generic integration. If not, ask them to.
- Share the infographic with your nursing staff. Have them hand it out during check-in.
It takes about 22 minutes of focused learning to overcome skepticism about generics. That’s less time than a coffee break. The payoff? Higher adherence, lower costs, better outcomes-and patients who trust you more.
Frequently Asked Questions
Are generic drugs really as safe as brand-name drugs?
Yes. The FDA requires generics to meet the same strict standards for safety, strength, quality, and performance as brand-name drugs. They undergo the same inspections and must prove bioequivalence-meaning they deliver the same amount of active ingredient at the same rate. In 2022, adverse event reports for generics and brand-name drugs were nearly identical.
Why do some patients think generics don’t work as well?
Many patients confuse packaging, color, or size with effectiveness. Others have heard outdated myths from older generations. Some are influenced by marketing that suggests brand-name drugs are superior. The FDA’s educational materials, including infographics and patient handouts, help correct these misconceptions by showing the science behind bioequivalence and manufacturing standards.
Can I trust generics made outside the U.S.?
Yes. The FDA inspects all manufacturing facilities-whether in the U.S., India, China, or elsewhere-that produce drugs sold here. Since the GDUFA law in 2012, inspections of overseas plants have increased by over 500%. The same quality controls apply. The drug’s origin doesn’t determine its safety-it’s the FDA’s approval process that does.
What about complex generics like inhalers or injectables?
These are more challenging to replicate exactly. For inhalers, topical creams, and some biologics, therapeutic equivalence isn’t always guaranteed. The FDA provides specific guidance for these products, and prescribers should review the labeling carefully. For most oral medications-pills and capsules-the science is clear: generics are equivalent.
How do I know if a generic is truly approved by the FDA?
All FDA-approved generics are listed in the Orange Book, which is publicly available. You can search by brand name or generic name. If it’s not listed, it’s not approved. Be cautious of online pharmacies or unregulated sources that claim to sell “FDA-approved” generics-they may not be. Always rely on prescriptions filled through licensed U.S. pharmacies.
Do generics take longer to work than brand-name drugs?
No. Bioequivalence testing ensures that the rate and extent of absorption are within the same range. If a brand-name drug starts working in 30 minutes, the generic will too. Any perceived delay is usually due to placebo effect or patient expectation-not pharmacology.
Is it okay to switch between different generic manufacturers?
Yes. All FDA-approved generics meet the same standards, regardless of manufacturer. Switching between different generic brands (e.g., from Teva to Mylan) is safe and common. The FDA monitors for any unexpected changes in performance, and no significant issues have been found in over a decade of tracking.
Next Steps for Prescribers
If you’re not already using FDA resources, start here: go to the FDA’s Generic Drugs website and download the Prescriber Flyer and Stakeholder Toolkit. Print the infographics. Share them with your team. Practice explaining bioequivalence in one sentence. The next time a patient asks why you’re switching them to a generic, you’ll be ready-and confident.
There’s no need to wait for your hospital to provide training. You have the tools. You have the data. You have the power to change prescribing habits-for your patients, your practice, and the system as a whole.






Inna Borovik
December 6, 2025 AT 22:52Let’s be real - the FDA’s bioequivalence range of 80-125% is a joke. That’s a 45% swing. If my blood pressure med dips below 80% of the brand’s effect, I’m going to stroke out. And don’t get me started on the overseas manufacturing. You think they’re inspecting every batch in India? Please. This is corporate cost-cutting dressed up as science.
Jackie Petersen
December 8, 2025 AT 14:14Generics are a scam. I’ve seen people switch and get worse. The brand name works. The generic makes them dizzy. The FDA doesn’t care about real people - they care about saving the insurance companies money. And now they want doctors to push this? No thanks.
brenda olvera
December 9, 2025 AT 12:38I love that the FDA made printable flyers and infographics for clinics 🙌 So many patients don’t understand the science but they do understand ‘same medicine cheaper’ - and that’s all we need to get them on board. My grandma switched to generic statins last year and she’s still kicking butt at 82. No drama. Just savings. Let’s make this easy for everyone.
Myles White
December 9, 2025 AT 21:56I’ve been prescribing generics for over 15 years and the data is rock solid. The only time I’ve seen issues is when patients are switching between multiple generic manufacturers back-to-back - which is rare and usually due to pharmacy stock issues, not drug quality. The real problem isn’t the generics, it’s the lack of integration into EHRs. Why should I have to manually pull up the Orange Book during a 10-minute visit? That’s not medicine, that’s administrative torture. If Epic can show me drug interactions in real time, why can’t it show me the $260 monthly savings on atorvastatin? It’s not a technical limitation - it’s a priority failure.
Brooke Evers
December 10, 2025 AT 14:27One of my patients told me she stopped her antidepressant because the generic made her ‘feel weird.’ I didn’t argue. I didn’t lecture. I just handed her the FDA handout and said, ‘Let’s try the brand this month - and next month, if you’re feeling stable, we’ll try the generic again, but this time with you in control.’ She came back three weeks later and said, ‘I think I was just scared.’ That’s the thing - it’s not about the science. It’s about trust. And trust is built when you let people feel heard, not corrected.
Chris Park
December 11, 2025 AT 06:32China and India produce 80% of U.S. generic active pharmaceutical ingredients. The FDA inspects less than 5% of these facilities annually. The rest are audited via paperwork. This is not regulation - it’s colonial negligence. The 80-125% bioequivalence standard is a mathematical loophole designed to legitimize foreign exploitation. The FDA’s own data shows 37% of generic recalls originate from overseas plants. This is not medicine. This is global supply chain gambling.
Saketh Sai Rachapudi
December 12, 2025 AT 03:28Why are we even talking about this? In India we give generics to millions and they work fine. You guys are overthinking this. The brand names are overpriced because of stupid marketing. People in the US think if it’s expensive it’s better. That’s why you’re sick all the time. Just take the generic and stop wasting money. Also the FDA is not perfect but they’re better than your local pharmacy who doesn’t even know what a bioequivalence is
joanne humphreys
December 13, 2025 AT 22:57I’ve been a nurse for 22 years and I’ve watched patients’ lives change when they can afford their meds. I’ve seen someone on insulin switch from $400 to $20 and stop choosing between groceries and shots. I’ve seen someone with hypertension stop missing doses because they weren’t drowning in debt. The science is clear. The tools are there. The only thing missing is time - and that’s something we can fix, one printed flyer, one calm conversation, one moment of patience at a time.