Bioequivalence: What It Means for Generic Drugs and Your Health
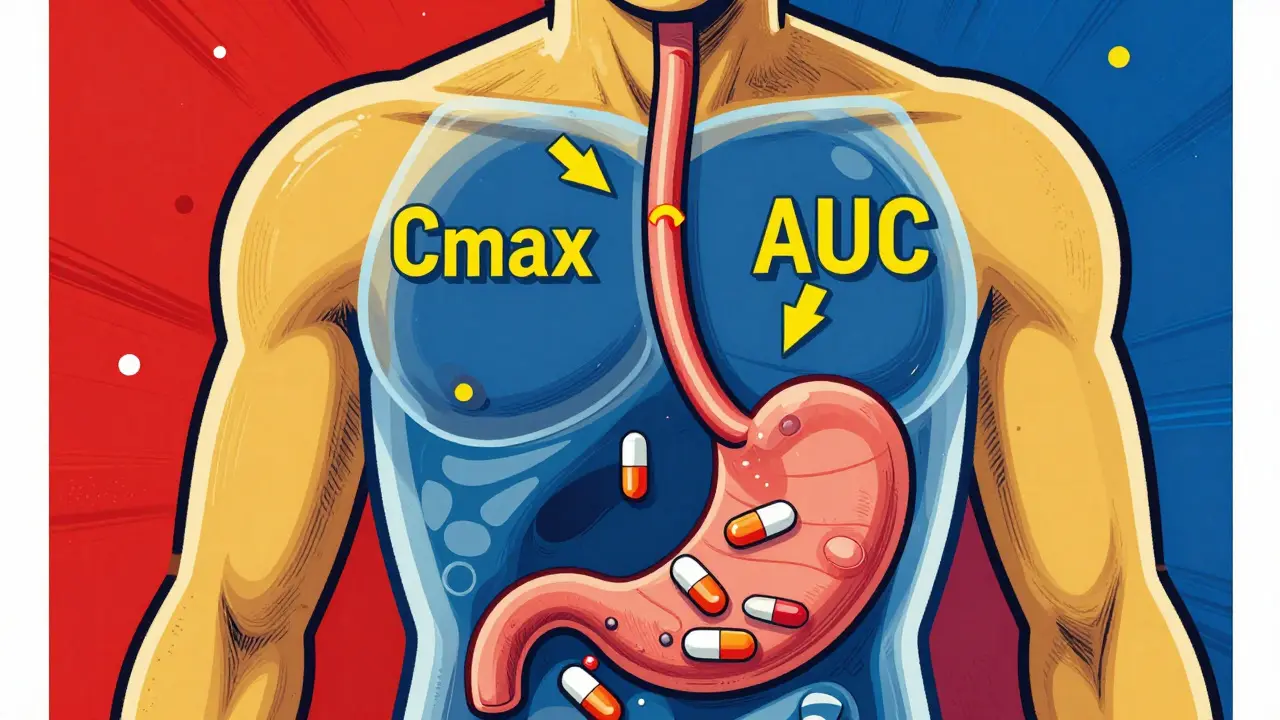
When you pick up a generic pill, you’re counting on it to do the same job as the brand-name version. That’s where bioequivalence, the scientific proof that two drug formulations release the same amount of active ingredient into your bloodstream at the same rate. It’s not just a buzzword—it’s the reason your $5 generic blood pressure pill works as well as the $100 brand. Without bioequivalence, generics could be useless—or worse, dangerous. The FDA doesn’t just approve generics because they’re cheaper; they require rigorous testing to prove they match the original in how your body absorbs and uses them.
This isn’t about looks or packaging. It’s about therapeutic equivalence, when two drugs produce the same clinical effect and safety profile in patients. drug absorption, how quickly and completely a medicine enters your bloodstream is the core metric. If a generic releases 85% to 125% of the active ingredient compared to the brand, within a narrow time window, it’s approved. That’s the sweet spot. Too slow? You won’t get relief. Too fast? You might overdose. This is why pharmacists can confidently swap generics without asking your doctor—because bioequivalence makes it safe.
But bioequivalence doesn’t mean identical in every way. Fillers, coatings, and shapes can differ—and that’s okay. What matters is the active ingredient’s journey through your body. That’s why some people notice a difference when switching brands: not because the drug failed bioequivalence, but because their body reacts to the inactive ingredients. Still, over 90% of patients see no change in results. And for the rest? Doctors and pharmacists track it closely, especially with narrow-therapeutic-index drugs like warfarin or thyroid meds.
Behind every approved generic is a mountain of data: blood samples taken at intervals, plasma concentration curves, statistical analysis. The FDA doesn’t rely on theory—they demand proof. And that’s why you can trust that your $3 generic statin works just as well as the brand, even if it looks different. This system saves the U.S. billions every year without sacrificing safety. It’s one of the most effective, science-backed cost-control tools in modern medicine.
What you’ll find in the posts below are real-world stories and facts about how bioequivalence shapes your access to medicine—from how pharmacists recommend generics to how counterfeit drugs try to sneak past these checks. You’ll see how FDA approval costs impact availability, how barcode scanning protects you from mix-ups, and why timing your calcium supplements matters when you’re on a generic antibiotic. These aren’t abstract concepts. They’re the invisible rules that keep your meds working, safe, and affordable.